[ad_1]
When it comes to the new COVID-19 vaccine, authorized for emergency use Friday by the U.S. Food and Drug Administration and given Monday to its first American recipients, the good news is very good indeed: U.S.-based Pfizer and its German partner BioNTech have managed to develop and deliver a shot that’s safe and 95 percent effective in less than a single year — roughly four times as fast as any previous vaccine. Other similarly promising COVID-19 vaccines will follow in the months ahead — including Moderna’s, which the FDA should authorize this week.
The less good news, however, is that millions of Americans — somewhere between 20 percent and 40 percent, depending on how the question is worded — still won’t commit to getting vaccinated, according to various polls.
Given that the United States has proven itself singularly unable to control the virus with masks and social distancing — new cases are now averaging 200,000 a day, with more than 2,400 daily deaths, the highest in the world — vaccines now represent the only plausible way out of this nightmare. The fewer Americans who get inoculated, the more who will die.
Yet despite widespread hesitancy, there are new signs of hope that “uptake” — the technical term for the proportion of the eligible population that receives a vaccination during a specific time period — will reach the threshold required to decisively slow transmission and effectively end the pandemic in 2021.
“Let’s say we get 75 percent, 80 percent of the population vaccinated,” Dr. Anthony Fauci, the nation’s top infectious disease expert, said last week. “If we do that, if we do it efficiently enough over the second quarter of 2021, by the time we get to the end of the summer, i.e., the third quarter, we may actually have enough herd immunity protecting our society that … we can approach very much some degree of normality that is close to where we were before.”
Here are four reasons why the reluctance that’s registering in polls now may not be a big problem in the end.
Story continues
Willingness to get vaccinated is already trending in the right direction
Wariness about COVID-19 vaccines is nothing new. Back in May, Yahoo News and YouGov asked Americans if they planned to get vaccinated against COVID-19. A full 45 percent said they wouldn’t get vaccinated (19 percent) or that they weren’t sure (26 percent).
Movement in these numbers is nothing new, either. Four months later, the Yahoo News/YouGov poll found that the share of Americans saying they wouldn’t get vaccinated (34 percent) or that they weren’t sure (33 percent) had swelled to more than two-thirds — a shift attributable to growing fears among Democrats and Independents that President Trump would successfully pressure regulators to approve an untested and potentially unsafe vaccine in order to boost his reelection effort.
But now, after the election and amid the rollout of the first real vaccine, the numbers are finally moving in the opposite direction.
According to Gallup, the fraction of Americans who said yes when asked if they would agree to be jabbed “right now” with a free “FDA-approved” vaccine rose from 50 percent to 63 percent between late September and late November, while the fraction who said no fell from 50 percent to 37 percent. (Technically speaking, the Pfizer vaccine was “authorized” for emergency use by the FDA, not “approved,” although that may happen eventually. The distinction is unlikely to matter to most laypeople.)
According to Marist, the fraction of Americans who said yes when asked if they would “choose to be vaccinated” when “a vaccine for the coronavirus is made available to you” rose from 49 percent in September to 61 percent in early December, while the fraction who said no fell from 44 percent to 32 percent. In both cases, 7 percent said they were “unsure.”
According to the Pew Research Center, the fraction of Americans who said they would “definitely” or “probably” get a “vaccine to prevent COVID-19” if it were “available today” rose from 51 percent in September to 60 percent in November, while the fraction would said they “definitely” or “probably” wouldn’t fell from 49 percent to 39 percent.
Likewise, a new Axios-Ipsos poll shows that the share of Americans who say they’ll get the COVID-19 vaccine as soon as it’s available has doubled since September — a shift driven by people over 65 yet evident across every demographic (age, party identification, race).
While in each case the raw number of yeses still isn’t as high as experts would like, the trendline — a net 20-point shift from wariness to willingness over the last two months — is encouraging.
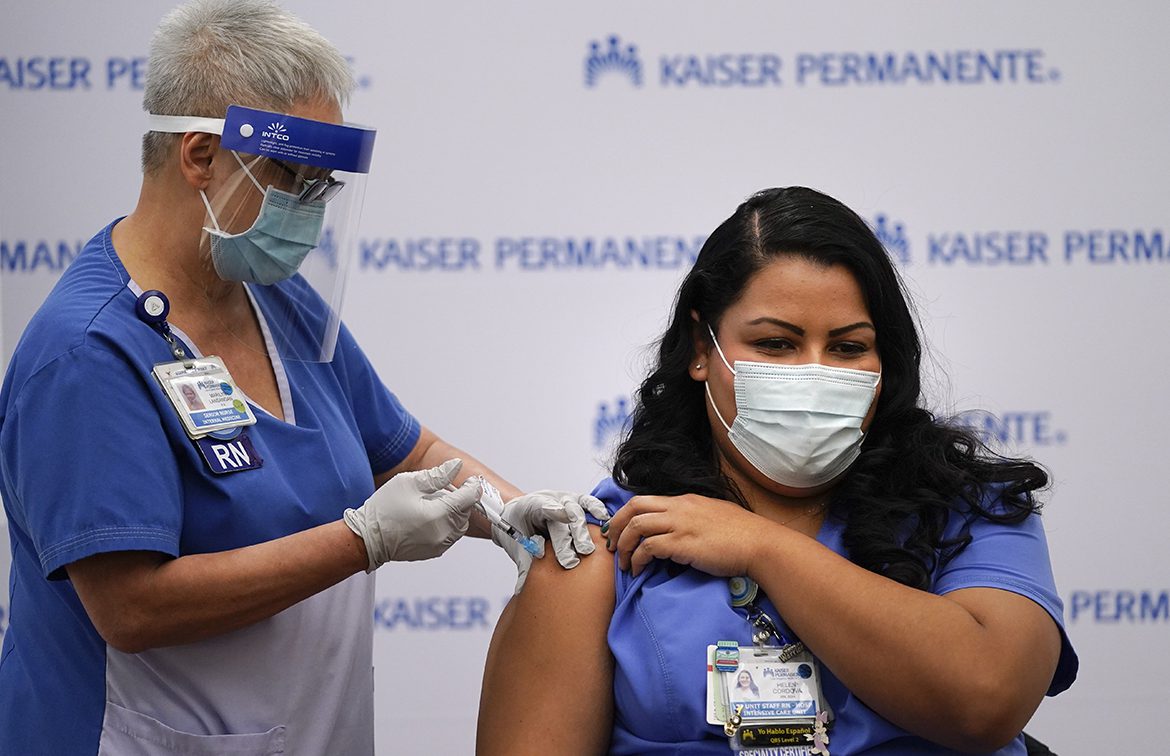
Nurse Helen Cordova, right, receives the Pfizer-BioNTech COVID-19 vaccine at Kaiser Permanente Los Angeles Medical Center on Monday. (Jae C. Hong/AP)
For one thing, it reinforces that public opinion is responsive to the latest developments around vaccines. For another, it suggests that public trust has been bolstered by recent developments: the leading vaccines’ impressive 90-to-95-percent effectiveness results in clinical trials, the so-far-successful introduction of the Pfizer shot in the U.K., and the conclusion of the presidential election, which has eliminated the suspicion that the FDA was under political pressure to rush its authorization.
More likely than not, this trust will continue to build as tens of millions of Americans — frontline health workers, nursing home residents, workers in other essential jobs — get uneventfully vaccinated, transforming COVID-19 immunization from a theoretical proposition into a proven passport back to normal, pre-pandemic life.
The rollout will take time — and time is exactly what hesitant Americans say they need
Earlier this month, a Quinnipiac University poll asked registered voters the same question as pretty much every other polling firm: “If a COVID-19 vaccine is approved by government health officials, do you think you would be willing to get vaccinated, or not?”
Similar to every other survey, 61 percent said yes and 33 percent said no.
Then Quinnipiac tried something a little different, giving respondents three options instead of two. “Which comes closest to your view?” the pollster asked. “You would take the COVID-19 vaccine as soon as it’s available to you; you would wait a few months to take the COVID-19 vaccine after it is available; or you would never take the COVID-19 vaccine.”
In response, just 20 percent of registered voters — the hardcore anti-vaccination crowd — said “never.” Poll after poll has pegged this group at about 15 to 20 percent of the population, and experts largely consider them a “lost cause.”
At the same time, however, 37 percent said they would get vaccinated as soon as possible; another 41 percent said they would prefer to wait a few months. That’s 78 percent in total. In other words, the share who said they would be willing to get vaccinated jumped 17 points (from 61 percent) simply because respondents were now given an option to choose to wait — presumably (and understandably) to see if the vaccine is safe and effective once it’s been administered to others.
Fortunately for these cautious types, they will have no choice but to wait a few months. By the end of 2020, about 20 million Americans will have been vaccinated. In January, Pfizer and Moderna are expected to start shipping enough doses to vaccinate 35 million people per month. If other companies in addition to Pfizer and Moderna receive approval for their vaccines, total capacity could reach 75 million people per month in March.
Up to that point, demand will almost certainly exceed supply: health professionals, nursing-home residents, essential workers and senior citizens have priority, in roughly that order. Each of these high-risk groups, which together add up to more than 100 million Americans, is likely to crave the protection these vaccines afford.
It will only be in the spring that less vulnerable Americans will finally have a chance to get vaccinated. The Quinnipiac poll suggests that after months of watching and waiting as others enjoy the benefits of inoculation, the vast majority of them will be ready to roll up their sleeves by then.
The side effects are real — but not unusual or scary
Pfizer’s COVID-19 vaccine is fairly “reactogenic” — meaning for many people, it comes with some drawbacks: arm pain, headache, chills, even fever.
These are all signs that the vaccine is doing its job: triggering an immune response that will produce the antibodies you need to shield you from the actual coronavirus.
The question is whether hearing more about these side effects will counteract the positive vaccine news and deter people from getting their first dose. (Both the Pfizer and Moderna vaccines require two doses for full immunity.)
It’s possible but unlikely. According to Pfizer’s clinical trials, 47 percent of participants reported feeling fatigued and 42 percent reported getting a headache after the first dose. Other than local pain (83 percent), these were by far the most common side effects.
That sounds like a lot. But here’s the thing: The fraction of Pfizer trial volunteers who received a placebo and reported the same symptoms wasn’t all that much lower — 33 percent for fatigue, 34 percent for headache.
Reports of gastrointestinal symptoms (vaccine: 12 percent, placebo: 11 percent), chills (vaccine: 14 percent, placebo: 6 percent), and achiness (vaccine: 21 percent, placebo: 11 percent) weren’t wildly different either. And just 4 percent of Pfizer vaccine recipients reported a fever after the first dose.
Compare that to the shingles vaccine, which induces achiness in 45 percent of recipients, chills in 27 percent, fever in 21 percent and GI symptoms in 17 percent.
A dose of the Pfizer COVID-19 vaccine is prepared at University of Wisconsin Health in Madison, Wisc., on Monday. (John Maniaci/Reuters)
In short, the first dose of Pfizer’s COVID-19 vaccine is well within normal boundaries in terms of side effects — a little more uncomfortable than your annual flu shot, a little less uncomfortable than a shingles inoculation. Tens of thousands of people have already received the Pfizer and Moderna vaccines in clinical trials, and none of them have reported any serious health problems. (After getting the Pfizer vaccine, two U.K. health-care workers with a “significant history” of allergies — both carried adrenaline autoinjectors, or EpiPens — developed symptoms of “anaphylactoid reaction,” an allergic response related to anaphylaxis that can be life-threatening. Such reactions can be treated with epinephrine, though people with serious allergies have been warned off of the vaccine). Normal side effects fade after a day.
It’s possible that even the relatively minor unpleasantness of the initial experience — along with reports of more intense symptoms after the second dose — will deter some people from returning for another jab a few weeks after the first. That would be unfortunate; one dose of the Pfizer vaccine is less effective in preventing COVID-19, though how much less is an open question.
Yet given the back-to-normal promise of full vaccination — a positive side effect that tens (or hundreds) of millions of other Americans will already be enjoying by next summer — it seems likelier that most vaccine recipients will consider the peace of mind that comes from finishing the job to be worth whatever fleeting pain a second shot entails.
Herd immunity could arrive sooner than expected
Dr. Fauci estimated that “75 percent, 80 percent of the population” would need to get vaccinated in order to achieve “herd immunity.” That aligns with the scientific consensus.
But while reaching 75 to 80 percent vaccine uptake should be America’s goal, it’s not unthinkable that the pandemic could be all but over before we get there.
Consider a new model called “Path to Herd Immunity” by Youyang Gu, an independent data scientist trained at the Massachusetts Institute of Technology who has proved to be one of the pandemic’s most accurate forecasters.
Gu estimates that about 17.3 percent of the U.S. population had already been infected with COVID-19 by Nov. 29. Forecasting from current trends, he projects that about 30 percent will have been infected by the end of March 2021.
This is important, because the latest data suggests that infection confers long-lasting immunity to COVID-19. If 30 percent of Americans already have this immunity — roughly 100 million people — then the percentage of Americans who need to acquire immunity through vaccination in order to effectively end the pandemic is probably a lot smaller than 75 or 80 percent.
Gu estimates that it’s closer to 30 or 40 percent — another 100 million people — and that the U.S. will hit this mark sometime around July.
In the interim, new daily COVID-19 infections (we only detect a fraction of these through testing) will have fallen from their current level of 550,000 to 300,000 in early February and 100,000 in late April before flatlining to around 5,000 in late June. “Deaths may drop to low levels even earlier (May-July 2021), in part due to a vaccine distribution strategy that initially prioritizes high-risk individuals,” Gu writes. “Once deaths fall to minimal levels, we may see a relaxation of restrictions.”
By the end of 2021, roughly two-thirds of the U.S. population will have been vaccinated against COVID-19, according to Gu’s model — not dissimilar from the number who already say they’re willing to get inoculated, with no need for additional persuasion.
The ultimate toll of the U.S. pandemic will be immense, Gu forecasts: somewhere around 500,000 American lives lost. But it could end, for all intents and purposes, long before 75 to 80 percent of the country gets vaccinated — if that many people ever get vaccinated.
Read more from Yahoo News:
[ad_2]
Source link