[ad_1]
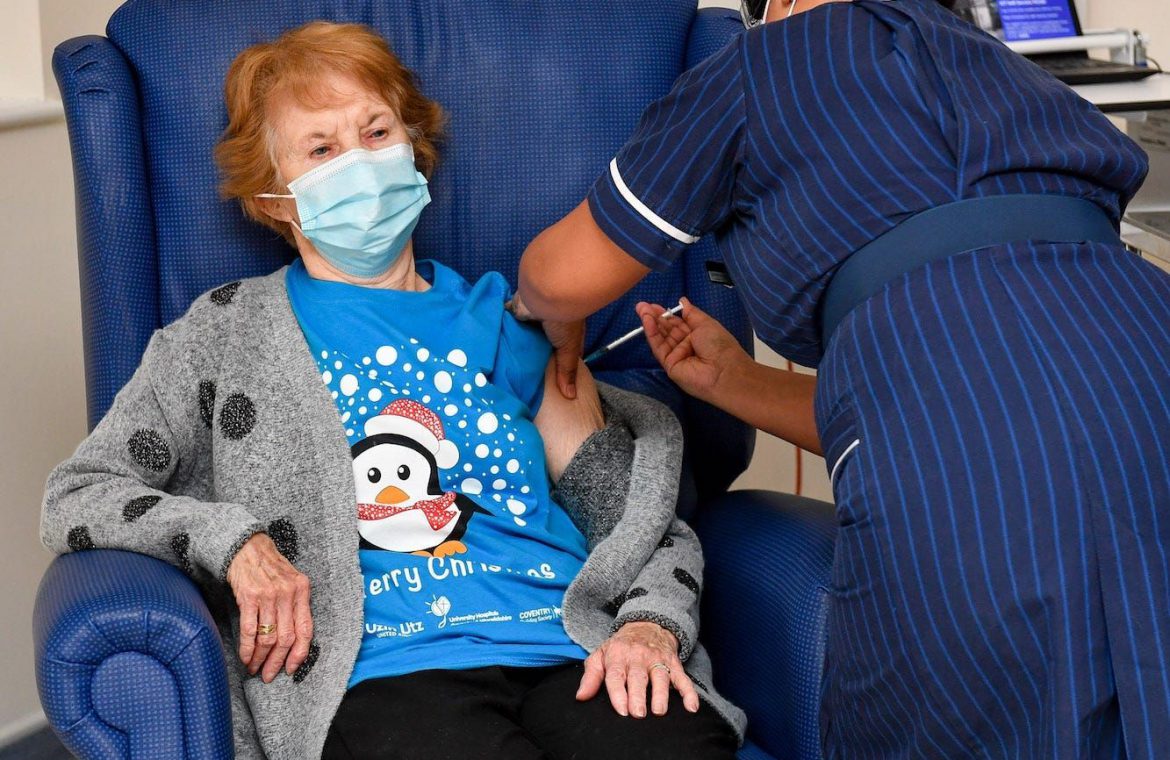
Margaret Keenan, the first person to be given the Pfizer coronavirus vaccine outside clinical trials. Getty
US vaccine regulators provided a detailed look at the side effects of Pfizer’s coronavirus shot on Tuesday.
Most trial participants reported temporary side effects after being vaccinated, including fatigue, headaches, and pain at the injection site. People under 55 reported more side effects overall, but relatively few participants had severe fatigue or headaches that stopped them from performing normal activities.
These results were consistent across genders, age groups, ethnic and racial groups, and people with preexisting medical problems. But several populations were excluded from the trials – meaning the vaccine isn’t known to be safe for all Americans just yet.
“There are currently insufficient data to make conclusions about the safety of the vaccine in subpopulations such as children less than 16 years of age, pregnant and lactating individuals, and immunocompromised individuals,” a recent Food and Drug Administration review concluded.
Some public-health experts have also questioned whether individuals who were already infected with the coronavirus should get vaccinated, particularly if they have lingering symptoms.
An FDA advisory committee is meeting Thursday to decide whether to recommend the vaccines for emergency use. But even then, these four groups will have to wait for more safety data.
Children under 16 are often last to be included in trials
Story continues
Kindergartner Grace Truax, 5, removes her mask before posing for a portrait during “picture day” at Rogers International School on September 23, 2020 in Stamford, Connecticut. John Moore/Getty Images
Research shows that many children clear the coronavirus before it leads to severe disease, resulting in mild or asymptomatic cases. Children only represent around 7% of confirmed coronavirus infections in the US – the lowest of any age group, according to the Centers for Disease Control and Prevention.
That means kids aren’t a priority group when it comes to vaccinations. They’re also difficult to include in trials due to ethical and safety concerns: Some parents may not feel comfortable signing their kids up to participate, and researchers often prefer to test vaccines in a small group of adolescents before enrolling young kids.
Pfizer’s clinical trials, for instance, did not include any participants younger than 12. Even then, the sample size of kids from ages 12 to 15 was too small to weigh the risks.
But there’s little reason to suspect that kids will react poorly to the shots.
“Children generally respond well to vaccines,” Donna Farber, a Columbia University immunologist, told Business Insider. “They should respond well or comparably to a young adult – and maybe even better.”
Pregnant women were excluded from trials, tooNancy Pedroza, 27, experiences contractions while taking a walk with her doula. REUTERS/Callaghan O’Hare
Pfizer’s phase-one clinical trial included primarily healthy men and women. Its late-stage clinical trials then tested the vaccine among more vulnerable populations, including healthcare workers and people with autoimmune diseases.
But the company excluded pregnant women. It asked female volunteers to take a pregnancy test before each vaccination, and any with positive results were not allowed to continue.
That means researchers don’t know how the vaccine affects pregnant women yet.
Pfizer plans to follow up with women who reported pregnancies during the course of the study, or those who choose to get vaccinated anyway once the shots are approved. That’s how public-health experts eventually determined it was safe for pregnant women to get the flu vaccine, even though these women were never included in flu vaccine trials.
Pfizer also plans to include pregnant women its vaccine study moving forward.
It’s important to get this data as soon as possible, since pregnant women are at high risk for COVID-19 complications. A November CDC report found that pregnant women with COVID-19 are more likely to be admitted into the intensive-care unit, put on ventilators, need life support, and die than patients who aren’t pregnant.
Pregnant women of color are particularly vulnerable: The report found that of all symptomatic pregnant women with COVID-19, 30% were Hispanic. But only 24% of women who gave birth in 2019 were Hispanic. White women, on the other hand, represented 51% of women who gave birth in 2019, but only 24% of symptomatic pregnant women with COVID-19.
HIV-positive individuals and others with weakened immune systems must also wait for more dataMichelle Grant hugs her mother, Mary Grace Sileo, through a plastic drop cloth in Wantagh, New York. It was the first time they’d physical contact since February. Al Bello/Getty Images
Though Pfizer’s late-stage trials included people with preexisting health problems, there wasn’t enough data to determine whether the vaccine is safe for those with weakened immune systems, according to the FDA review. For example, the trials only included a small number of HIV-positive individuals, and researchers didn’t have enough time to follow up with this group before applying for emergency authorization.
It’s a good sign that serious adverse events were only reported in 0.6% of vaccine recipients, but immunocompromised people have to be particularly careful about vaccines. If a patient’s immune system isn’t working properly, they may require extra doses. Others might want to avoid getting vaccinated while taking immunosuppressants like corticosteroids or chemotherapy drugs.
For the most part, doctors recommend that immunocompromised people steer clear of vaccines that use live virus. But Pfizer’s vaccine instead relies on a snippet of the virus’ genome to trigger an immune response.
Dr. Edward Jones-Lopez, an infectious-disease specialist at the University of Southern California, told Healthline that he estimates “we are one or two studies away” from finding out whether coronavirus vaccines are safe for immunocompromised people.
People who previously got COVID-19 are in a grey area Patient Setsuko Smidmore awaits testing at St George Hospital in Sydney, Australia, May 15, 2020. Lisa Maree Williams/Getty Images
Pfizer hasn’t specified whether its vaccine is safe for people who previously had COVID-19. Studies suggest these patients may have immunity to the virus that lasts anywhere from several months to several years. But the research is far from conclusive. They’re likely to need a vaccine at some point down the line.
“We want to look at vaccinating patients who have not been infected with COVID who are susceptible,” Todd Ellerin, director of infectious diseases at South Shore Health in Massachusetts, told Business Insider. “The post-COVID patients are not going to be your first, second, third, or fourth tier of groups that you’re going to look at to want to vaccinate.”
For people with long-lasting symptoms – known as long-haulers – it’s possible that a vaccine could intensify an already aggressive immune response that for many has led to fatigue, muscle aches, trouble breathing, heart palpitations, or difficulty concentrating or sleeping.
“It’d be hard to engage a long-hauler in [a vaccine] study if they’re having ongoing health problems,” Natalie Lambert, an associate professor of medicine at Indiana University, told Business Insider. “Ethically there’d be big problems with having them take a vaccine, if you’re worried about those effects.”
Pfizer, however, didn’t screen participants for evidence of a prior coronavirus infection during its late-stage clinical trial. So it turned out that 3% of participants had been infected before. The data showed that the vaccine was just as effective among this group, but the FDA review said there wasn’t enough evidence to know if the vaccine prevented reinfection.
Ellerin said it’s unwise to inoculate previously infected people without more safety data.
“We have to keep our eye on the prize right now,” he said. “Of course patients can get COVID more than once, so I’m not trying to tell you that they’ll be excluded from this down the road, but we have a lot more to learn.”
Read the original article on Business Insider
[ad_2]
Source link