[ad_1]
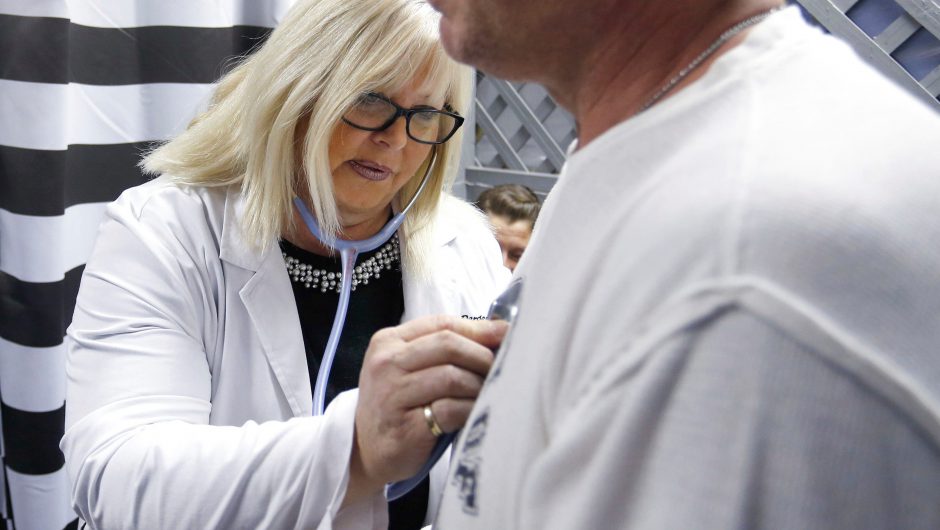
A blood donor in Bangkok, Thailand, lays on a transfusion chair. The Thai Red Cross has requested to donate blood plasma from patients recovering from COVID-19.
Amphol Thongmueangluang/SOPA Images/LightRocket/Getty Images
The FDA is considering authorizing convalescent plasma as an emergency treatment for COVID-19.
The therapy involves treating sick patients with the blood of those who have already recovered.
Convalescent plasma is also the basis for a drug, hyperimmune globulin, which would deliver consistent antibody levels to patients.
But researchers still need to prove that the treatments are safe.
Visit Business Insider’s homepage for more stories.
During the 1918 Spanish flu pandemic, doctors discovered they could treat sick patients with the blood of those who had already recovered. The therapy, known as convalescent plasma, helped reduce mortality among people with acute infections. Now, it’s showing promise as a treatment for COVID-19.
That’s because many people who get the coronavirus develop neutralizing antibodies — our body’s natural response to a foreign pathogen. Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, has called them “the gold standard of protection against a viral infection.” Since antibodies develop in plasma, the liquid portion of blood, doctors can transfer this plasma intravenously to a patient.
Related video: What we know about immunity for the coronavirus
If the treatment works, the new patient would also mount an antibody response to the virus.
“What we really need are drugs that, when given early, can prevent a symptomatic person from requiring hospitalization or very dramatically diminish the time that they’re symptomatic,” Fauci told Facebook founder Mark Zuckerberg last month.
Convalescent plasma is especially promising in that regard — in theory, it could prevent a mild case from becoming more severe.
Already, the US Food and Drug Administration is allowing more than 2,700 hospitals to administer the treatment through an expanded access trial led by the Mayo Clinic. As of Monday, the trial has delivered plasma to more than 90,000 patients, according to the program website.
Story continues
The FDA is now getting closer to authorizing convalescent plasma for emergency use, according to the Wall Street Journal. An emergency use authorization would allow doctors to administer the treatment earlier in the course of an infection, when it’s believed to be most effective.
However, there are still major limitations to its widespread use. Plasma must be transferred quickly from a donor to a recipient — and both must have compatible blood types. The quantity is also limited, since it depends on blood donations. That means plasma isn’t likely to be a long-term treatment for the virus. Instead, researchers and pharmaceutical companies see it as an interim therapy until a vaccine becomes readily available.
Early research shows promiseA recovered coronavirus patient donates blood samples for plasma extraction to help critically ill patients at the National Blood Transfusion Center on June 22, 2020.
Ameer Al Mohammedaw/Getty
Preliminary studies on convalescent plasma have primarily focused on its efficacy among hospitalized patients.
A May study from researchers at Mount Sinai — which is still awaiting peer review — found that hospitalized coronavirus patients who received convalescent plasma had a higher chance of survival than those who didn’t receive the treatment: Only 18% of the plasma recipients saw their conditions worsen two weeks after their transfusion compared to 24% of patients who didn’t receive the treatment.
By May 1, nearly 72% of the plasma recipients had been discharged from the hospital compared to 67% of the other patients. But the study was small — only 39 patients received transfusions — and the results were only significant for patients who didn’t need a ventilator.
In another study (also not yet peer reviewed), researchers at the Mayo Clinic found that convalescent plasma reduced the mortality rate among hospitalized coronavirus patients by 57%.
Mayo Clinic researchers also determined that the treatment was relatively safe among 5,000 adults with severe or life-threatening cases. Less than 1% of the patients developed severe side effects within four hours of receiving a blood transfusion. Although transfusions always pose some risk to seriously ill patients, only four deaths were linked to the plasma therapy.
Drug companies are converting plasma into medicineAn Iraqi phlebotomist holds a bag of plasma donated by a recovered COVID-19 patient at the blood bank of Nasiriyah in Iraq’s Dhi Qar province, June 24, 2020.
Asaad Niazi/AFP/Getty Images
In May, a coalition of medical institutions, drug companies, nonprofits, and COVID-19 survivors launched “The Fight Is In Us” — a campaign to get more recovered coronavirus patients to donate blood. Some of that blood will be used for direct transfusions, and the rest will go toward manufacturing hyperimmune globulin, a drug built from convalescent plasma.
The process of creating hyperimmune globulin involves pooling plasma from recovered patients and heat-treating it so that any remaining pathogens get destroyed. The result is a vial of medicine with consistent antibody levels that can easily be administered to patients. The drug focuses on the most common antibody found in blood — immunoglobulin G (IgG) — which usually confers long-term immunity.
“It’s basically treated in a way that reduces the likelihood that it could pass on any infections,” David Reich, president and chief operating officer of The Mount Sinai Hospital, told Business Insider in April. “Something like that could be helpful, potentially, to people in the early phase of the disease or potentially as prophylaxis against the disease.”
Mount Sinai is working with Emergent BioSolutions, a Maryland-based biopharmaceutical company, to develop a hyperimmune globulin product. The group will study whether the drug can protect individuals at high risk of exposure, such as healthcare workers, from getting infected in the first place.
A coalition of 10 drug companies involved in the “The Fight Is In Us” campaign is also creating a hyperimmune globulin drug. The research is led by Takeda, Japan’s largest pharmaceutical company, and CSL Behring, a Pennsylvania-based biotech company. They’re hoping to determine whether hyperimmune globulin improves outcomes for coronavirus patients with severe cases.
“Our goal here isn’t to continue to produce hyperimmune globulin ad infinitum,” Christopher Morabito, head of research and development for plasma-based therapies at Takeda, previously told Business Insider. “Our goal here is to have an effective therapy to bridge us to a point where either the pandemic is over because it dies out, or because there’s a vaccine available, or until there are many more effective treatments for patients with this disease.”
The coalition hopes to secure regulatory approval from the FDA by the end of 2020. But there are plenty of hurdles: Drug quantities are still limited by the quantity of plasma people donate. Companies researching hyperimmune globulin and direct convalescent plasma treatments also still need to prove that the approaches are safe.
As of Friday, Fauci said health officials were “still analyzing the data” to see if convalescent plasma is effective for COVID-19.
Read the original article on Business Insider
[ad_2]
Source link